Mannitol and Sorbitol for biological drugs formulation
Polyols are versatile sugar alcohols, also defined as sugar-free sweeteners low in calories.
They can be derived from both natural and synthetic sources. Natural sources of polyols include vegetable oils, fruits and starch for example.
Polyols are very resistant to heat, acidity, alkalinity, as well as enzymatic and chemical breakdown. These assets make them great candidates to be used as formulation excipients for the stabilization of biological APIs.
Mannitol and Sorbitol, hexahydric alcohols related to mannose, are the leading polyols consumed. Indeed, they were respectively present in 20,9% and 5,2% of the biologics formulations in 2020. [1] They are used as excipients mainly for monoclonal antibodies, vaccines and lyophilized formulations. Both have great solubility in aqueous solutions, sorbitol is slightly less soluble than mannitol.
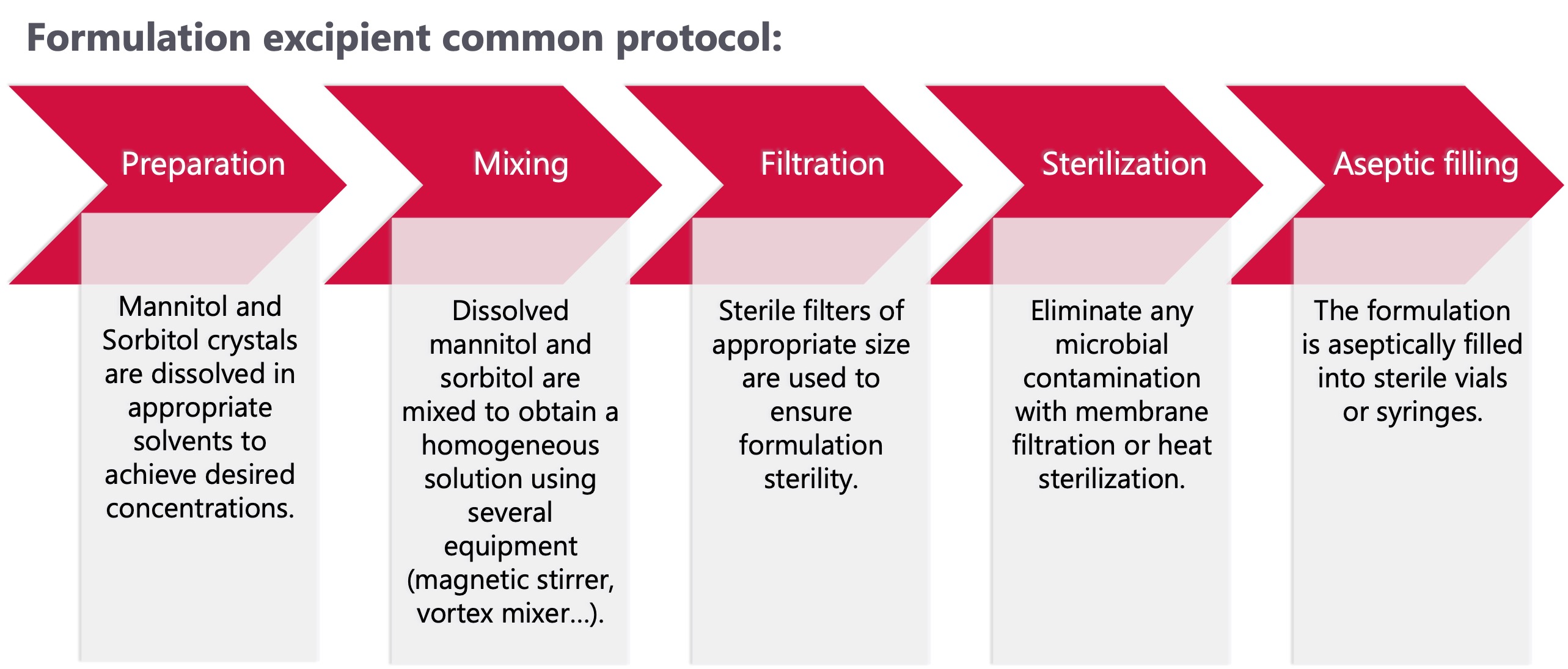
Interested in using mannitol and sorbitol in your formulation:
-
Bulking agents: prevent blowouts by increasing the cake mass during freeze-drying processes
-
Tonicity modifying agents: avoid osmotic shock at the site of injection.
-
Stability enhancers: prevent degradation reactions, such as hydrolysis or oxidation.
-
Carrier: enhance the dissolution rate of drug substances
In order to find a perfect formulation for biologics of interest these two polyols can be mixed with other products including stabilizers (sugars, amino acids), buffers (phosphate, acetate, citrate), surfactants (polysorbate 20, polysorbate 80), preservatives (phenol), tonicity adjusting agents (sodium chloride, glycine, sucrose), chelating agents (EDTA, citric acid), and antioxidants (ascorbic acid, alpha-tocopherol).
[1] (Ionova & Wilson, 2020)
Why use mannitol and sorbitol
Why use mannitol and sorbitol as a major excipient rather than the conventionally used sucrose?
-
Patient tolerance: lower reported incidence of allergic reactions, low fermentability, and less likely to cause gastrointestinal discomfort
-
Osmotic pressure: sucrose has a higher osmotic pressure than mannitol. A high osmotic pressure can cause water to be drawn out of cells, potentially leading to cell shrinkage or protein denaturation.
-
Crystallization: sucrose has a tendency to crystallize (at higher concentrations or during long-term storage) however, it can lead to changes in the physical properties of the formulation and may affect product stability.
Quality requirements
To maintain the stability, efficacy and quality during the formulation process, mannitol and sorbitol both require a biopharma low endotoxin grade. According to the European Pharmacopoeia, the limit for endotoxins in injectable-grade mannitol is 2.5 International Units (IU) of endotoxins per milligram (IU/mg) and of 0.5 IU of endotoxins per milligram (IU/mg) for injectable-grade sorbitol.
Sources:
Ionova, Y., & Wilson, L. (2020). Biologic excipients : Importance of clinical awareness of inactive ingredients. PLOS ONE, 15(6), e0235076. https://doi.org/10.1371/journal.pone.0235076
Lang, K., Sánchez-Leija, R. J., Gross, R. A., & Linhardt, R. J. (2020). Review on the Impact of Polyols on the Properties of Bio-Based Polyesters. Polymers, 12(12), 2969. https://doi.org/10.3390/polym12122969
Thakral, S., Sonje, J., Munjal, B., Bhatnagar, B., & Suryanarayanan, R. (2023). Mannitol as an Excipient for Lyophilized Injectable Formulations. Journal of Pharmaceutical Sciences, 112(1), 19‑35. https://doi.org/10.1016/j.xphs.2022.08.029
Get in touch
Contact us for more information and support in your biologics development process!
 e-mail us
e-mail us

